Rare Anaemia Disorders European Epidemiological Platform
The Challenges
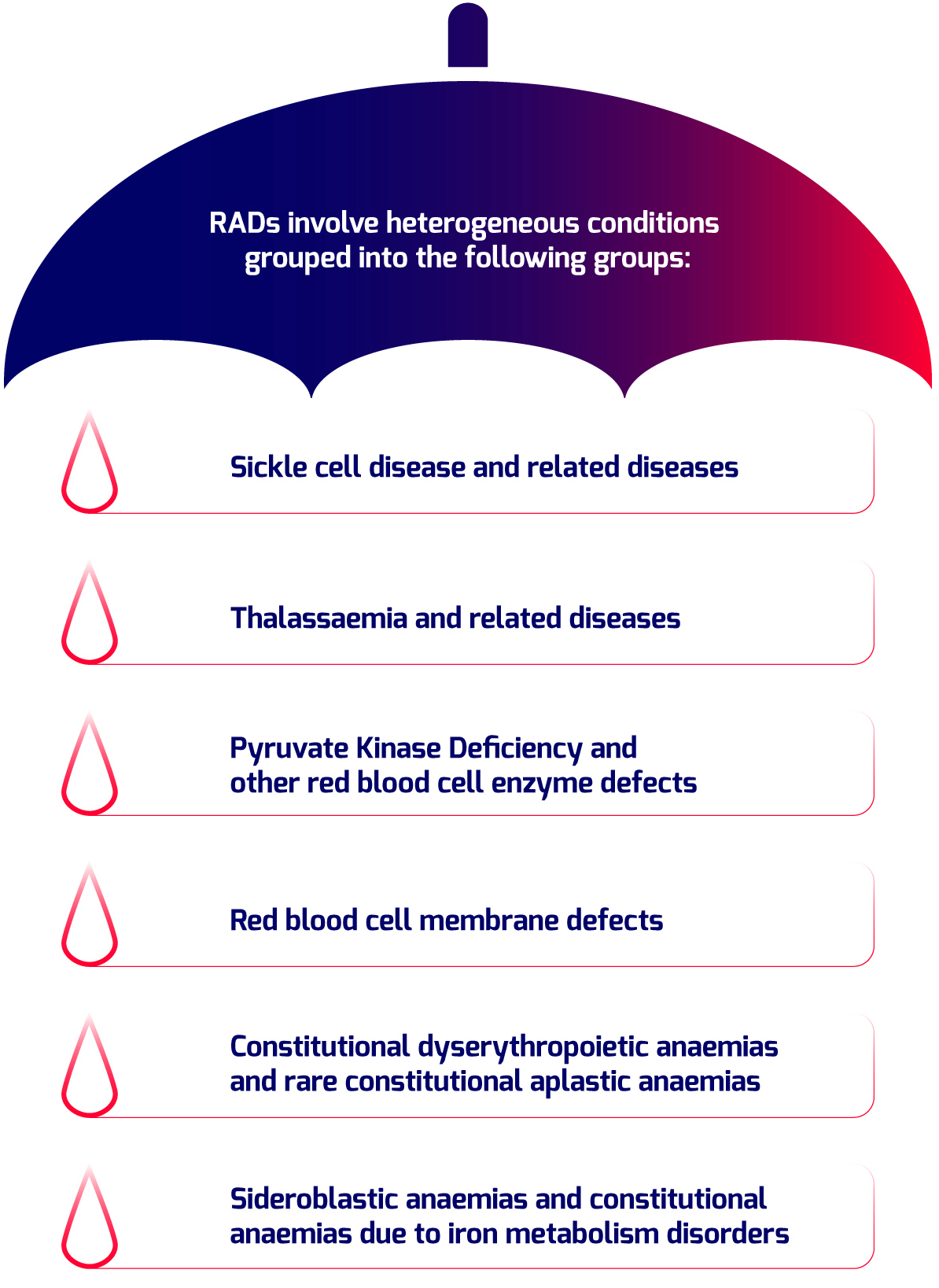
Rare Anaemia Disorders (RADs) is a group of rare diseases characterized for presenting anaemia as the main clinical manifestation. Different medical entities classified as RADs by ORPHA classification are most of them chronic life threating disorders with many unmet needs for their proper diagnosis and clinical management that requires from multidisciplinary teams on centers of expertise, creating an impact on European health systems.

Although there are some examples of well-established national registries on RADs in EU, the lack of recommendations for Rare disease registries implementation and the lack of standards for interoperability has led to the fragmentation or unavailability of data on prevalence, survival, main clinical manifestations or treatments in most of the European countries, required for both shapping policies and promotion of research.
RADeep Objectives & Outcomes

What is our solution?
RADeep is an initiative endorsed by ERN-EuroBloodNet for a European approach for the standardised collection of data regarding the main clinical complications of RADs.
How is RADeep going to implement the solution(s)?
- Secure access and re-use of health data: Establishment of a legal frame for RADeep secure processing and re-use of Findable, Accessible, Interoperable, Reusable (FAIR) Data with third parties. RADeep contributes to European Rare Blood Disorders Platform (ENROL) as the ERN-EuroBloodNet registry for rare haematological diseases.
-
Avoid fragmentation of data:
RADs patients' data collected from any EU country and sources available (ie. existing
registries,
hospital records) promoting interoperability in line
with the EU RD Platform
by the implementation of
- Data Set for RD patients
- International codification schemes ORPHA, ICD, SNOMED and HPO
- Pseudonymisation tool GDPR-compliant
- Engage community to promote -OMICS based research on drug-able targets and clinical trials: Allow the identification of groups of patients with confirmed diagnosis of RAD and with common demographics and primary clinical disease manifestations.
- Improve access to specialized clinical management of RAD patients: Promoting best practices and access to adequate diagnosis methods while fostering the creation of guidelines and external quality assessment for RAD.
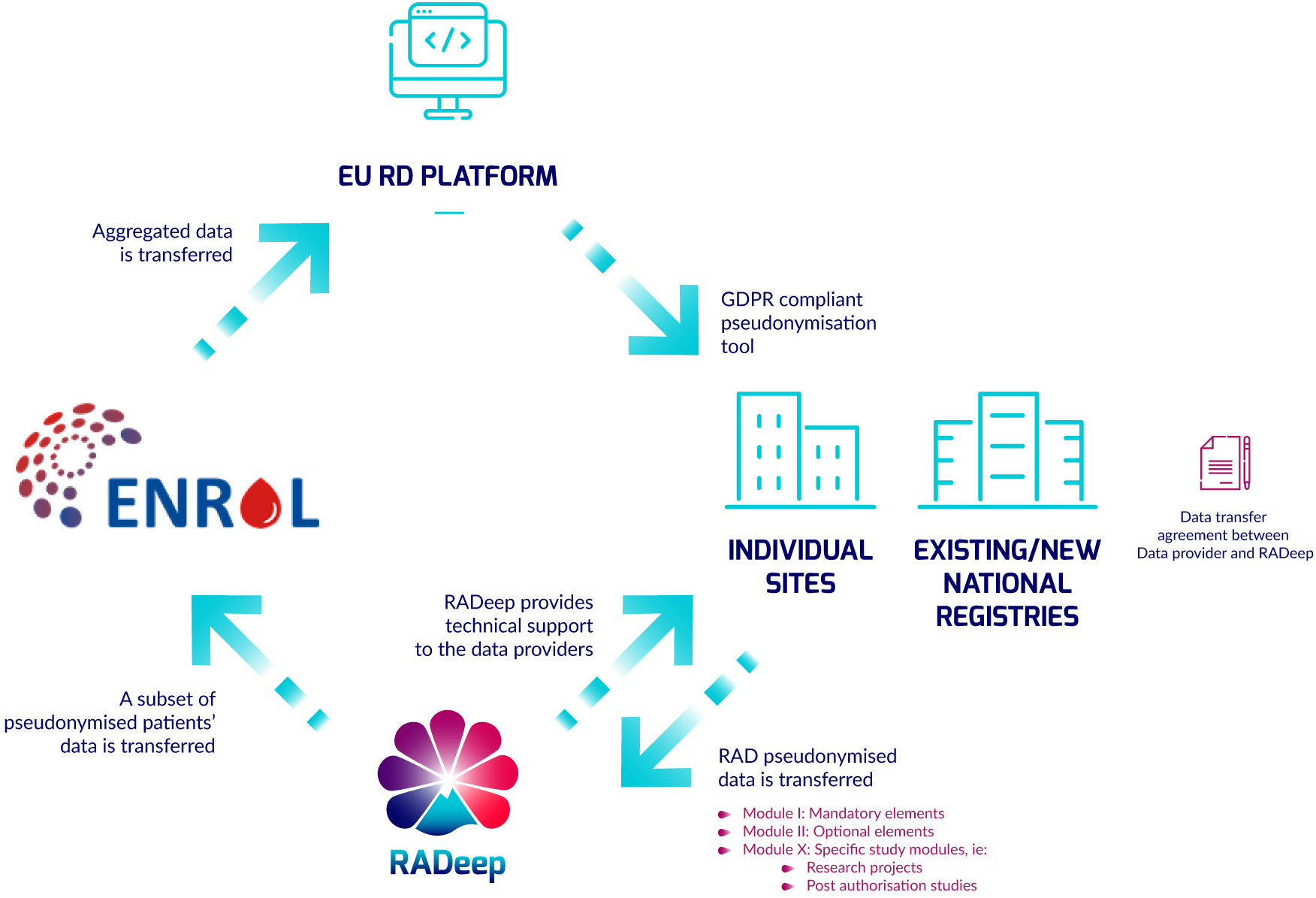
RADeep Dataflow
The RADeep dataflow is a ring-shaped process through EU RD Platform, individual sites and existing/new registries, RADeep and ENROL.
- A subset of pseudonymised patients' data is transferred from RADeep to ENROL.
- Aggregated data is transferred from ENROL to EU RD Platform.
- The EU RD Platform provides a GDPR compliant pseudonymization tool to individual sites and existing/new registries.
- RAD pseudonymized data is transferred to RADeep from individual sites and existing/new registries.
- At the same time RADeep provides technical support to the data providers.
RADeep acts as the umbrella for both already existing and new registries on any
RAD in EU, aiming at avoiding fragmentation of data
by promoting the standards for interoperability
while ensuring patients’ rights for EU cross border share of
data.
Consortium
RADeep is led by a Consortium formed by:

|
Coordinator
Maria del Mar Mañú Pereira Research Institute (VHIR), Barcelona, Spain - Vall d’Hebron University Hospital (HUVH) - Vall d’Hebron University Hospital Foundation |

|
Béatrice Gulbis
Erasme University Hospital (ERASME) / LHUB-ULB, Brussels, Belgium |

|
Marina Kleanthous
The Cyprus Foundation for muscular dystrophy research (CING), Nicosia, Cyprus |
|---|